A few people report dramatic improvement after taking one of these experimental drugs. At the same time, a few people have also gotten dramatically worse on these drugs. ![]()
Nucleoside analogues
Nucleoside analogues are molecules that are really similar to the building blocks used to make RNA (or DNA). When these analogues (lookalikes) bootleg their way into the RNA being produced, they throw a wrench into the whole process and screw up the production of new viruses.
The nucleoside analog inhibits the viral RNA-dependent RNA polymerase (RdRp) by competing with the usual counterpart adenosine triphosphate (ATP). The nucleoside analog is incorporated into the generating RNA strand and causes a delayed stop in the viral replication process.
A Review on Remdesivir: A Possible Promising Agent for the Treatment of COVID-19 - PMC
History of remdesivir / ‘remdeathsivir’
Remdesivir was tried as a treatment for Ebola. In one study, four different therapies were tried. Remdesivir shared last place with another drug.
The data and safety monitoring board found higher mortality in the ZMapp and remdesivir groups compared to the MAb114 and REGN-EB3 groups.
The journey of remdesivir: from Ebola to COVID-19 - PMC
Later on remdesivir was tried for the coronavirus (SARS-CoV-2). A large randomized controlled trial by the WHO found remdesivir to be ineffective. The WHO actually recommended AGAINST its use. In the DisCoVeRy trial in France, the investigators argued that 1-3 people were killed by remdesivir.
However, many other health organizations endorsed its use. ¯\__(ツ)_/¯
Because the emergency drug approval process was driven by money rather than good medical practices, regulators ignored remdesivir’s mechanism of action. It interferes with actively replicating virus and doesn’t do anything to dead virus or viral debris. It’s only in the early stages of COVID that the virus is still replicating. When the virus is still alive and still replicating, there is a window of opportunity to attack the virus with antivirals (e.g. remdesivir) so that less viral debris will be produced. Gilead, the drug’s manufacturer, was well aware that the drug should work best when it is given as early treatment.
Gilead funded a number of early treatment clinical trials and was figuring out how to deliver the drug earlier, e.g. using methods that are more convenient than IV. Unfortunately, these trials must have turned out badly because Gilead cancelled its early treatment trials and killed off that early treatment pipeline. This is extremely awkward because it strongly suggests that the drug causes more harm than good when used as early treatment. Nonetheless, Gilead manipulated the trial results and now most countries recommend using remdesivir as early treatment. Because this is how healthcare works.
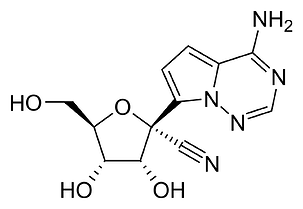
GS-44, or GS-441524
Remdesivir is a “pro-drug”. Your body will break it down into its metabolites, one of which is called GS-441524. GS-441524, or GS-44, is the molecule that actually does something (“active ingredient”).
So basically, GS-44 and remdesivir are fairly similar. In practice there may be some subtle differences between the two.
Where remdesivir and GS-44 are useful
Both molecules are useful against FIP or Feline Infectious Peritonitis which is caused by a coronavirus that infects cats. Whereas FIP is a lethal disease, remdesivir has a good chance of helping somebody’s pet cat survive.
There is a black/grey market for remdesivir and GS-44. If you put the right search terms into Google, you will find advertisements for these drugs.
John Chia uses remdesivir for ME/CFS patients
John Chia believes that enteroviruses cause ME/CFS. See his website, Health Rising, or this interview for more information on the enterovirus theory. His own son suffered from ME/CFS (well before the pandemic started) and he successfully treated his son with oxymatrine (and likely other treatments). His son now works for Gilead, which happens to be the company that owns remdesivir.
After remdesivir became available, Chia started experimenting with it for ME/CFS patients. There’s not much information on patient outcomes but a few sources talk about what he’s doing such as this Health Rising article.
ME/CFS patients have tried GS-44
GS-44 is not approved as a drug for humans or cats. It is not commercially available through legitimate means. However, because the molecule is public knowledge, anybody can get the drug custom synthesized for them. So that’s what ME/CFS patients did. This video sort of explains how you might get access to GS-44… if you really want to know.
GS-44 patient outcomes
Unfortunately, there hasn’t been a good systematic effort to report the results of this human experimentation. What I can tell you is a few people who tried it got a lot better and a few people got a lot worse (one went from being able to work to not being able to work; that person developed a vax injury after ME/CFS). It may have been 3 people who got better and 3 who got worse (with multiple others about the same), but my memory may be wrong.
Nucleoside analogues have the potential to be useful???
The obvious problem with remdesivir+GS-44 is that it has killed some COVID patients and it seems to cause worsening in chronic illness patients at higher rates than killing the patient. So I’m definitely not recommending either drug.
However, as we learn more, perhaps we may discover a safe way of using these drugs (e.g. starting with low dosages, discontinuing early). We might also look at other nucleoside analogues like Truvada Emtricitabine / Tenofovir (which I talk about in this video) to see if they might be useful for chronic illness.
I hope you find this interesting and please don’t do stupid things with the drugs mentioned.