Their three main studies are:
- Recovery Study: NCT04895189 - the effect of vaccination on unvaxxed LC patients. Yale COVID-19 Recovery Study
- PAX LC: NCT05668091 - Paxlovid as a treatment for LC. The website explains the study.
- LISTEN study conducted on the Hugo Health Kindred platform
The Recovery Study has been a troubled study that has been repeatedly pushed back. Its completion has been pushed back 3 years (!) from about May 2022 to May 2025. There is a good chance that the researchers are sitting on the results for political, non-scientific reasons.
The PAX LC is funded by Pfizer:
Pfizer provided funding for the study and also participated in the design development.
Pfizer’s involvement in the trial design suggests that the study will be biased to make Paxlovid look good.
Many members of the chronic illness communities are participants in the LISTEN study so there are questions as to whether or not the science will be good. So let’s dive into it and look at each study one by one…
Recovery Study
This study has probably been plagued by recruitment issues. This is not surprising because there are 2 big limitations that reduce the potential pool of participants:
- The participant had to be within driving distance of Yale’s lab. So that’s a small fraction of the US population that can participate.
- The participant had to be unvaxxed AND willing to be vaccinated.
It makes sense that the researchers had to push back the completion when they started realizing that recruitment may be tough. The study has cycled through a lot of people, starting with Dorothy Massey, then César Caraballo-Cordovez, and then nobody / back to Harlan Krumholz.
The problematic part is where this study stopped recruiting and the completion was pushed back 2 years from May 2023 to May 2025. This is all public information on ClinicalTrials.gov. I suspect that there is no scientific rationale for pushing back the release date. As recruitment has stopped, I would presume that the trial did not hit its original enrollment target (which is fine); the researchers can simply release what they have.
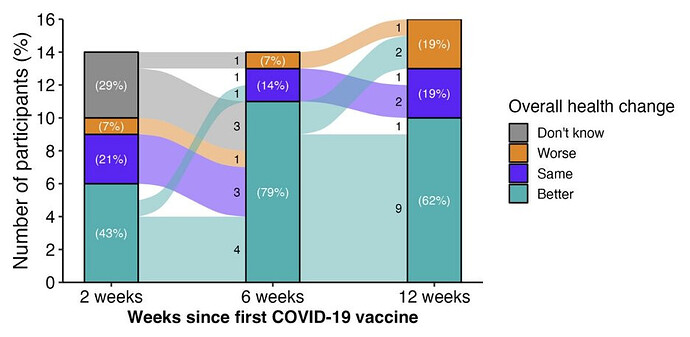
We can speculate as to what the results of the trial were. Other similar studies suggest that COVID vaccinations cause more harm than good in chronic illness patients (see the slides in the video description for this video). A straightforward guess would be that this study found that vaccinations cause a little more harm than good. But because it’s science, we shouldn’t just assume that the results are what we expect them to be. Unexpected findings are quite common in science. One might guess that the results were highly unanticipated and pose political problems for the researchers for some reason… which would explain why they aren’t releasing their findings.
PAX LC
This study was funded by Pfizer. Pfizer played a role in this trial’s design development and it shows.
The study website explains why ritonavir will be given to the control group:
The placebo includes ritonavir, which does not have an effect on the virus, but will help ensure people don’t know whether they are in the Paxlovid or placebo group.
This rationale is suspect. The main clinical trial used to approve Paxlovid was EPIC-HR, whose results have been published in the NEJM. That trial did not give ritonavir to the control group (e.g. read the paper or see NCT04960202). The EPIC-HR paper didn’t seem to mention any issues with the trial’s blinding.
It is probably easier for Paxlovid to outperform ritonavir than it is for the drug to outperform a placebo pill (assuming perfect blinding of participants that works). Ritonavir would introduce unnecessary side effects. Making the control group take ritonavir lowers the bar for Paxlovid. This would be useful if LC patients regularly did a course of ritonavir by itself. But they don’t. So the trial results would not generalize to real-world LC patients, who have no reason to take ritonavir by itself.
Drug interactions
The trial also excludes people who are taking common drugs that may interact with Paxlovid. Because many LC patients take drugs that would interact with Paxlovid, the trial results would not necessarily generalize to those patients. Ideally, there would be a trial where such patients are included and their medications changed temporarily to adjust for Paxlovid’s effects.
Participants can’t get any vaccinations for 28 days
For some reason, the study excludes people who plan to get vaccinated (with a COVID or non-COVID vaccine). Whoever designed the trial presumably expects an interaction between either:
- Vaccination and Long COVID ← The LC community may have better health outcomes if this were disclosed to them.
- Vaccination and Paxlovid (in people with LC). No such interaction is disclosed in the FDA label for Paxlovid.
LISTEN study
Those participating in the LISTEN study may be interested in knowing what’s going on with the other studies.
-
The non-publication of the Recovery Study is a potential issue as non-publication of results can create a publication bias in the scientific literature. It can cause the published scientific literature to be biased and skewed in ways that can lead people astray. Perhaps vaccines are a highly beneficial or harmful treatment for Long COVID. The LC community should be informed about those results.
-
The design of the PAX LC study raises issues about the conflict of interest between the researchers and their funding sources (e.g. Harlan Krumholz and Akiko are running both PAX LC and LISTEN). While Pfizer did not fund the LISTEN study, the results of the LISTEN study or the Recovery Study may be delayed to make funders happy. NCT05668091 shows that the FDA grant/contract 2U01FD005938-03 is now associated with the study.
So perhaps LISTEN participants could get some answers so that they know how their data will be used. Will their participation be wasted or co-opted to favour a funder’s political agenda? Will the study results be published in a timely fashion?
Other Yale studies
Yale research teams have completed other studies such as the study on myocarditis caused by either mRNA vaccine and a deep profiling study on Long COVID patients (collaboration with Mount Sinai).
Some of the findings in those studies go against the researchers’ financial interests. For example, Aaron Ring is the inventor of the REAP auto-antibody panel. His published research suggests that the panel is not useful for the treatment of vaccine-induced myocarditis.
The Ring / Akiko Iwasaki / Barmada et al. group has put out research on vaccine injury (myocarditis) that doesn’t have any issues with the published research being tainted by potential conflicts of interest (e.g. GAVI, Bill and Melinda Gates Foundation, WHO, NIAID/NIH, CDC, etc.).