Famotidine is a histamine H₂ receptor antagonist medication that decreases stomach acid production. In healthy people, it is generally considered to be fairly safe as it is sold over-the-counter.
For chronic illness patients, you can think of famotidine (Pepcid) as a lower-risk bandaid ![]() that may offer quick symptom relief. It seems to be more effective in patients with severe food intolerances. Unfortunately, it likely does not deal with the root cause and it likely does not help patients recover.
that may offer quick symptom relief. It seems to be more effective in patients with severe food intolerances. Unfortunately, it likely does not deal with the root cause and it likely does not help patients recover.
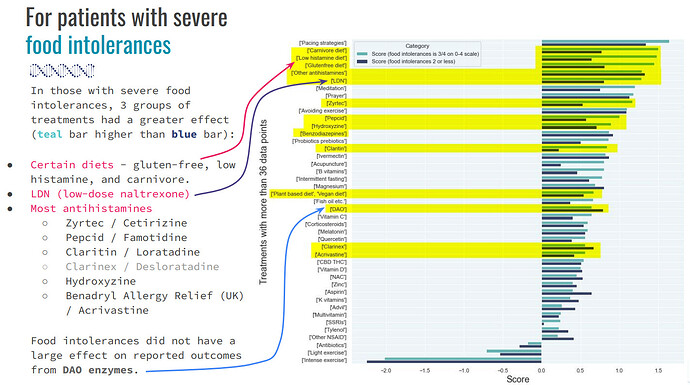
Works better in patients with severe food intolerances (or allergies)
The slide below is from the Nov 2022 data-driven video. It highlights three groups of treatments that work better in those with severe food intolerances or allergies- diet, antihistamines, LDN.
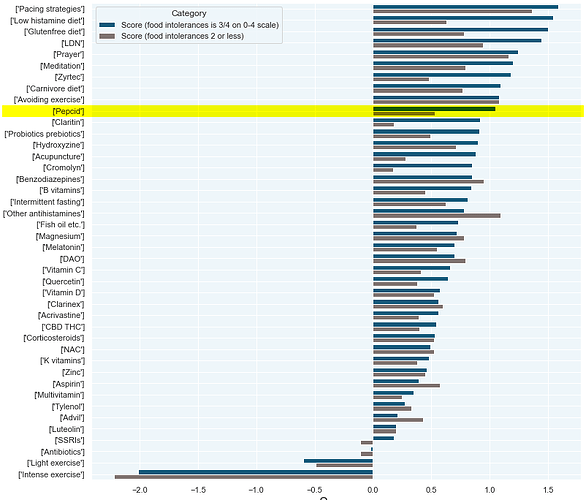
Here is the same data again with a slightly larger sample size:
Treatment Outcomes Survey analysis 2023-07-12
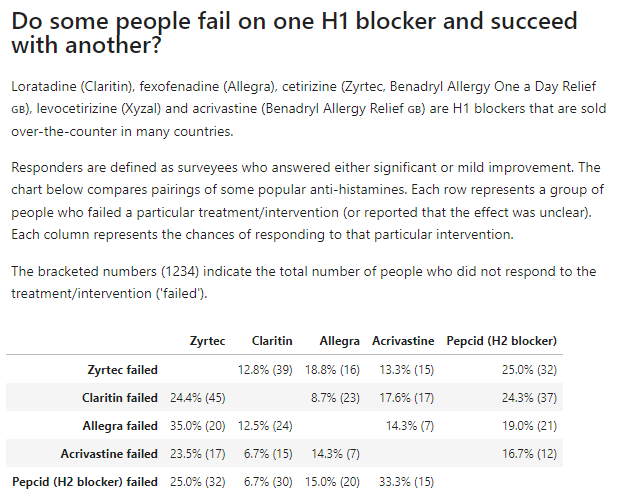
For those who do not respond to famotidine, there is a chance (1-in-3 or less) that they will respond to other antihistamines.
Risks and safety
Of the 54 most popular treatments, Pepcid is #20th highest in terms of its risk score. (See this post for an explanation of risk score.) It has a higher risk score than ivermectin, which is #26.
Treatment Outcomes Survey analysis 2023-07-12
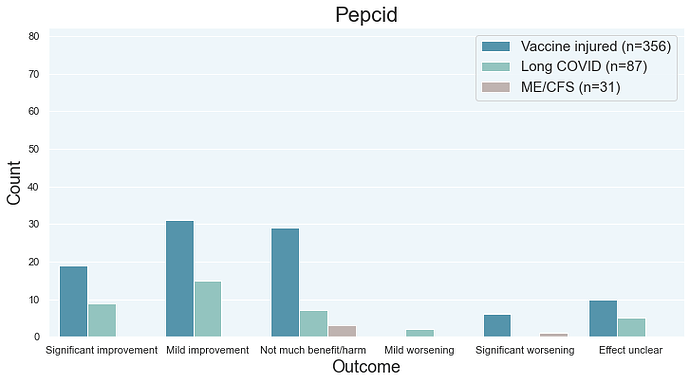
A handful of post vac patients reported significant worsening from famotidine/Pepcid. Some Long COVID patients reported significant worsening from other antihistamines so it would be reasonable to assume that Long COVID patients can also experience significant worsening from Pepcid.
There doesn’t seem to be anecdotal reports of long-lasting side effects from famotidine but I could be wrong.
Drug interactions
I haven’t researched this, sorry.
Does it deal with the root cause of chronic illness?
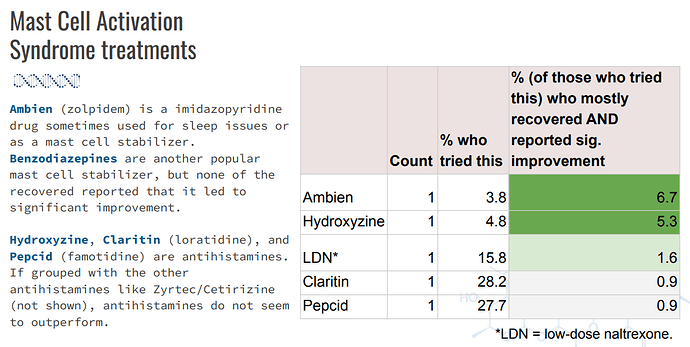
Data is from the Treatment Outcomes Survey (from the what worked video). In general, MCAS treatments don’t seem to help people recover.
If all of the antihistamines are pooled together, then less than 1% of the surveyees will be mostly recovered AND reporting significant improvement from antihistamines. Ivermectin is at 4.3% while multi-day fasting is at 19%.
Antihistamines were fairly popular treatments. For famotidine specifically, about 28% of the Treatment Outcomes Survey participants reported that they tried it. The numbers below won’t match the numbers above because I didn’t have severity data for all of the survey participants.
Randomized Controlled Trial data
An Iranian team lead by Momtazmanesh (DOI:10.1016/j.jpsychores.2023.111389) studied patients who were recovering from COVID patients at a care center after discharge. While the study found that famotidine improved psychiatric symptoms (the authors work in the psychiatry field), I have some concerns with the research.
- The patients don’t seem to be “Long COVID” patients. Of the 98 post-hospitalization patients who were assessed for eligibility, 58 were randomized into the trial. This cohort may not necessarily reflect the type of Long COVID where patients experience a debilitating syndrome characterized by a multitude of symptoms.
- Shahin Akhondzadeh, a major member of the research team, has been involved in multiple RCTs that have found a benefit for the intervention tried. Akhondzadeh’s consistency in research success is unusual as the primary endpoints in his studies typically reach statistical significance. See his Long Haul Wiki page for a list of his RCT studies and key portions of the abstracts.
I’m not convinced that the RCT is reliable and relevant for chronic illness patients.
Other clinical data
Glynne and colleagues (DOI:10.1136/jim-2021-002051) studied a cohort of Long COVID patients (along with a convenience sample of vaccinated healthy controls). Their prospective observational study found some improvement from histamine receptor antagonists (antihistamines) - famotidine plus either loratadine or fexofenadine.
What I don’t like about observational studies is that there is an ‘everything works’ phenomenon in the scientific literature. We shouldn’t throw all of it out but a lot of it is unreliable. This particular study was not randomized or blinded, so it’s theoretically possible that the study is just measuring the biases of people who want to take antihistamines for at least 4 weeks versus people who don’t want to do that. There is also the possibility of the placebo effect doing something.
Recap
Famotidine (Pepcid) is among the lower-risk treatments so I don’t think the potential downside is that bad. It’s not a big deal if you experiment with it.
It’s worth trying if your food intolerances or allergies are severe (close to the worst suffering imaginable).