Bettina Hohberger and her colleagues have published the results of their RCT on BC007. The full paper is here.
According to them:
Rovunaptabin [BC007] showed a neutralisation of GPCR-fAAb and a significant improvement of
FACIT Fatigue Scale (effect size = 2·10, p = 0·0378),
Bell score (effect size = 3·64, p = 0·0004),
Fatigue Severity Scale (effect size = −2·66, p = 0·0088),
and quality of life (4/8 items).
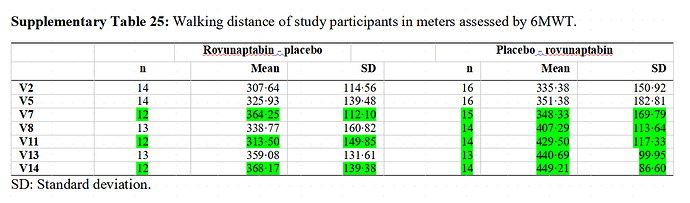
Possible issue with the data
They weren’t able to get questionnaires and walking test results on every visit. That’s fine. People drop out of studies… that’s just the reality of running studies.
However, they present data where partial results are mixed into the overall data. This does not make sense.
To get Supplementary Table 25, go to https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(25)00290-1/fulltext → Supplementary material (4) → Suppl. Tables and Figures
The partial data can inflate or deflate the mean/average whenever it is included.
I don’t know if they did this for the data that they cite in the abstract.
Other data issues
The abstract cherry-picks the secondary endpoints which make BC007 look good. I’ll list all of the secondary endpoints and bold the ones that are mentioned positively in the abstract:
- Mean change and cross-over differences
- of fatigue symptoms determined using the
o Bell scale (V2 [day 0; baseline] vs. V7 [day 28] or V8 [day 42] vs. V13 [day 70])
o Canadian Criteria (V2 [day 0; baseline] vs. V7 [day 28] or V8 [day 42] vs. V13 [day 70])
o Chalder Fatigue Scale (V2 [day 0; baseline] vs. V7 [day 28] or V8 [day 42] vs. V13 [day 70])
o FACIT Fatigue Scale (V2 [day 0; baseline] vs. V7 [day 28] or V8 [day 42] vs. V13 [day 70])
o FSS (Fatigue Severity Scale) (V2 [day 0; baseline] vs. V7 [day 28] or V8 [day 42] vs. V13 [day 70]) - Quality of life as measured by the SF-36 questionnaire (V2 [day 0; baseline] vs. V7 [day 28] or V8 [day 42] vs. V13 [day 70])
- exercise capacity determined by the 6-MWT (walking distance, Borg scales); (V2 [day 0; baseline] vs. V7 [day 28] and V8 [day 42] vs. V13 [day 70])
- Microcirculation based on vessel density in the capillary plexus of the macula and/or around the optic nerve in the OCT-A scan (V2 [day 0; baseline] vs. V7 [day 28] and V8 [day 42] vs. V13 [day 70]).
Qualitative determination of functional GPCR autoantibodies (detectable/not detectable) (V1 [screening], V5 [day 7], V7 [day 28], V11 [day 49], V13 [day 70])
Occurrence of AEs, ARs, SAEs and SARs in the entire observation period (baseline to V14 [day 90])
Source: the secondary endpoints were pre-registered at Clinical Trials Register
If you use the Chalder fatigue scale and 6-MWT (walking distance test), then the results would go the other way- placebo would outperform BC007. This is more clearcut with the Chalder fatigue scale.
But the real issue is that partial data was mixed into the results. The supplementary materials don’t seem to present data without that questionable methodology.
FACIT doesn’t measure fatigue?
You can find the FACIT questions here.
Some of the questions seem problematic for the BC007 study if the goal is to measure fatigue:
- “My family has accepted my illness”
- “I am satisfied with my sex life”
If you participate in a trial, the participation may affect the family’s acceptance of the illness. (And I don’t think LC patients / post-COVID syndrome patients would view the family dynamic as being related to fatigue.)
Commentary from somebody who participated in the Erlangen trial by Bettina Hohberger and colleagues
Please, don‘t get up your hopes on BC007! Thats all I need to say.
https://www.reddit.com/r/cfs/comments/1mluv4v/a_personal_statement_as_trial_participant_to/
*I don’t necessarily agree or disagree with the Reddit user’s commentary. The press articles are interesting as one of them states that the drug helps.